Abstract
BACKGROUND Cancer-associated VTE is a significant cause of morbidity and mortality. The Khorana score (KS), which includes cancer type, body mass index, and prechemotherapy laboratory studies, is commonly used to stratify the risk of cancer-associated VTE (1). KS has low discriminative ability in some clinical situations (2) and does not include genomic information. We investigated a cohort of 753 pts with PC for germline and somatic predictors of VTE and splanchnic thrombosis and developed a PC-specific polygenic risk score (PC-PRS) to predict VTE.
METHODS We manually abstracted clinical risk factors for VTE in pts of European ancestry with PC, sequenced at Dana-Farber Cancer Institute (DFCI). VTE was defined as 1) thrombosis in deep veins of the lower extremities/upper extremities or 2) pulmonary embolism. Splanchnic thrombosis was separately analyzed and defined as thrombosis of the portal, hepatic, splenic, or superior/inferior mesenteric veins. Incident VTE/splanchnic thrombosis was defined as the first occurrence of thrombosis or thrombosis-related death. KS was calculated using pretreatment laboratory values obtained prior to cancer treatment.
A genome-wide association study (GWAS) was conducted for time to VTE across 5.35 million common imputed germline variants. A germline PRS was computed by cross-validation: 1) the cohort was split into 5 subsets; 2) GWAS was conducted within 4/5 subsets and PRS weights calculated using pruning and thresholding; 3) each score was predicted into the held out 1/5 subset; 4) this was repeated five times; 5) significance of the association between held-out PRS and VTE was evaluated by permutation. Associations between both PC-PRS and KS and time to VTE were determined. To assess PRS and GWAS specificity, we conducted an association analysis of 5760 solid tumors sequenced at DFCI and treated with anti-neoplastic therapy for which VTE information was available.
Pathogenic somatic variants in tumor suppressor genes included truncating variants, splice-site variants affecting consensus nucleotides, or homozygous deletions. For proto-oncogenes, pathogenic calls included gene amplifications and missense variants with functional impact determined using SIFT(3) and Polyphen-2(4). False discovery rate was applied using Benjamini Hochberg (FDR<10%)
RESULTS Of 753 pts with PC, 305 (40.5%) underwent PC surgery and 661 (87.8%) received chemotherapy within 6 months (mo) of diagnosis. During a median follow-up of 4.4 years, 223 (29.6%) incident VTEs and 94 (12.5%) splanchnic thromboses were identified. For 529 pts with available KS, 131 (24.8%) were intermediate-risk while the remaining were high-risk.
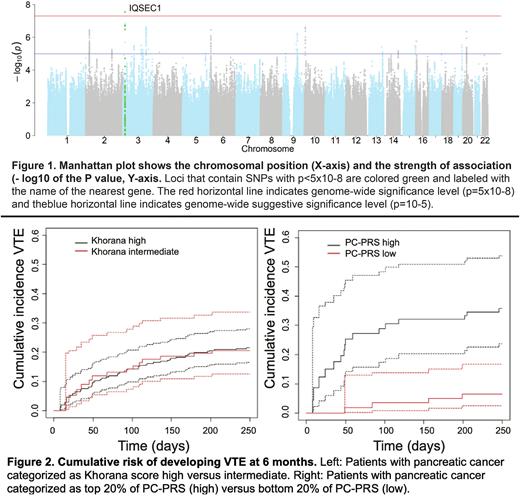
GWAS across 753 samples identified 1 locus that was genome-wide significant for VTE (p<5x10-8). The lead SNP was rs13327332, with the minor allele associated with a significantly lower risk of VTE (HR=0.13, p=2.8x10-8, fig 1) in the PC cohort. The variant was not associated with VTE in other solid tumors.
To investigate genome-wide polygenic effects, a risk score was estimated using all GWAS SNPs and evaluated by cross-validation (PC-PRS). Individuals in the top 20% of PC-PRS were at 5-fold higher risk for developing a VTE in the first 6 mo relative to the lowest 20% of PC-PRS, after accounting for the competing risk of death. Cumulative incidence of VTE in the first 6 mo was similar between pts with intermediate and high KS (fold-change=1). After adjusting for sex, age, chemotherapy within 6 months, history of prior VTE, surgery for pancreatic cancer, metastatic disease at time of VTE, and tumor purity, PC-PRS was significantly associated with time to incident VTE (HR=1.9, p=0.02) while KS was not (HR=1, p=0.96, fig 2). The PC-PRS was specific to PC and was not associated with other solid tumors.
Analyzing 445 genes for associations between pathogenic somatic alterations and time-to-VTE, CNV amplifications in CIC were associated with significantly higher risk of VTE (HR=2.5, p=0.0001, FDR<10%). MAP3K1 gene alterations (n=12) were associated with 9.3x higher risk of splanchnic thrombosis (p=0.0001, FDR<10%).
CONCLUSION We suggest a role for PC-PRS in VTE risk stratification and identify multiple somatic alterations with high effects on VTE incidence. Implementation of PC-PRS and integration with somatic data may provide a more refined risk estimation of VTE risk in PC than current clinical tools permit. Further validation in independent cohorts is warranted.
Disclosures
Connors:Roche: Honoraria, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alnylam: Consultancy; Abbott: Consultancy, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Research Funding; Anthos: Membership on an entity's Board of Directors or advisory committees; Werfen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.